Date Range:
Publication Date:
Owner/Creator:
Contact:
Metadata provider:
Methods:
Experimental methods: These data were collected in the lowlands of watersheds 4A and 4B at Konza Prairie Biological Station. In 2015, we collected leaf gas exchange and water potential from six shrubs in the lowlands of 4B. We sampled five ramets equidistant from the periphery of the clone to the center of the clone on six sampling dates throughout the growing season. In 2018, we collected leaf gas exchange, leaf isotopes, and leaf functional traits from the periphery and center of 20 shrubs located in the lowlands of 4B and 20 shrubs located in the lowlands of 4A. 4A was burned in April of 2018 and we measured resprouting shrubs that experienced 100% aboveground mortality after fire.
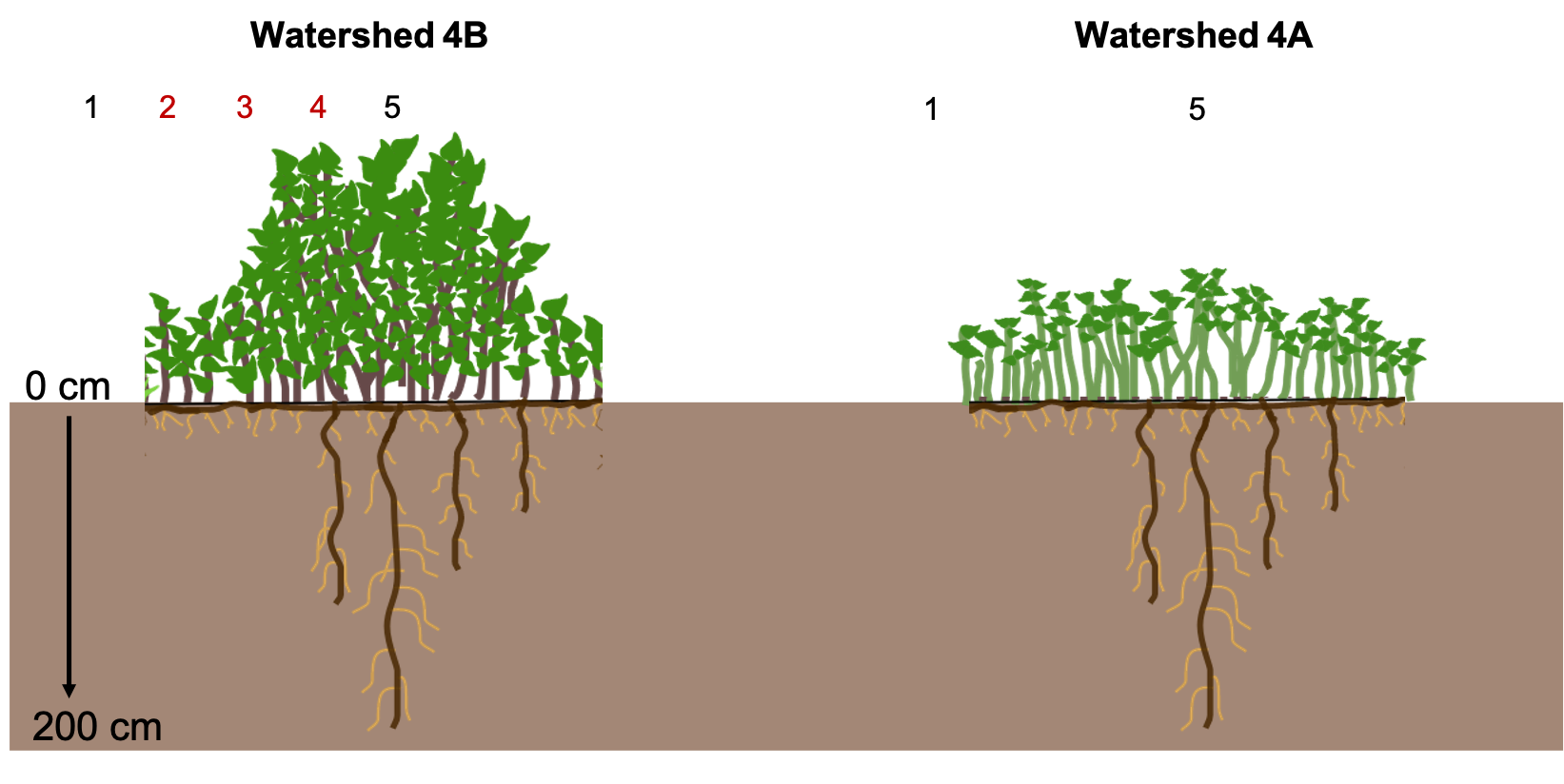 Figure 1. Diagram of sampling locations within C. drummondii shrubs. We sampled shrubs within watershed 4B in 2015 and 2018. Red numbers indicate ramet locations that were only sampled in 2015. In 2018, we sampled resprouting shrubs in watershed 4A.
Figure 1. Diagram of sampling locations within C. drummondii shrubs. We sampled shrubs within watershed 4B in 2015 and 2018. Red numbers indicate ramet locations that were only sampled in 2015. In 2018, we sampled resprouting shrubs in watershed 4A.
Data collection methods:
• Shrub Area: Shrub canopy area was estimated using an ellipse area equation by measuring the length of the longest axis and its perpendicular width through each discrete shrub.
• Gas Exchange: Instantaneous gas exchange rates were taken using a Li-6400XT open system gas analyzer (Li-Cor, Inc., Lincoln, NE). In 2015, gas exchange measurements were taken on 5 ramets within each shrub clone six times throughout the growing season (May-September). In 2018, gas exchange measurements were taken on one stem on the periphery and one stem in the center of each shrub four times throughout the growing season. Gas exchange measurements were collected between 10:00 and 15:00 h.
• Water Potential: In 2015, predawn and midday water potentials were measured six times throughout the growing season. Leaves for predawn water potentials were collected approximately one hour prior to dawn and leaves used for midday measurements were collected at approximately 12:00 h. Leaves were equilibrated for one hour in a dark, moist, high [CO2] plastic bag to ensure stomatal closure. Leaf water potential was measured using a Scholander pressure chamber (PMS Instrument Company, Albany, OR).
• Carbon isotopes: Four young, fully expanded leaves from the center and four leaves from the periphery of each shrub were collected for stable isotope analysis. Leaves were dried at 60℃ for 72 hours. For each shrub, the leaves from the periphery and center were each combined and ground. We measured stable carbon isotopic composition at the Kansas State University mass spectrometry lab. See Nippert et al. 2013 for a similar protocol.
• Leaf traits: Leaf traits were measured on the same leaves collected for carbon isotope analysis. We measured leaf area of fresh leaves using the LEAFSCAN smartphone application (leafscan.com). We then dried the leaf tissue at 60℃ for 72 hours and subsequently weighted leaf dry mass.
For additional metadata information see: http://lter.konza.ksu.edu/sites/default/files/DC.pdf
For additional methods information see:: http://lter.konza.ksu.edu/sites/default/files/MM.pdf
Maintenance:
complete



